NAFDAC also warned that such chemicals, aside from causing cancer, could lead to psychiatric and kidney problems. It equally warned against the continued use of banned chemicals by unscrupulous cosmetics manufacturers.
Reading what seemed like a riot act in Lagos during the first NAFDAC’s Good Manufacturing Practice, GMP, training for cosmetics and medical devices manufacturers in Lagos, Director-General of the agency, Dr. Paul Orhii, said the use of glutathione intravenous as a skin whitener was unsafe and might result in serious consequences, including cancer.
Continue to read
He expressed concern that the urge to satisfy customers’ ever-increasing demands and the bid to make profits had driven some cosmetics manu-facturers into experimenting with raw materials with little or no safety records.
Orhii vowed that the agency would not hesitate to shut down any company, which failed to comply with good manufacturing practice or caught with the banned chemicals.
He said:
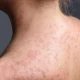
“Skin bleaching is a cosmetic treatment
to reduce the prominence of skin discolourations and even out skin colour.
“Some people apply skin lightener to their
entire body to change their complexion, but this can be very risky. The active ingredient in some skin lighteners is mercury and so bleaching can lead to mercury poisoning.”